From the first phases of research to the distribution of the finished product, the creation of life-saving drugs is a difficult and meticulous procedure. Imagine a future in which, as a result of the relentless efforts of pharmaceutical industry scientists and producers, illnesses that are today crippling or life-threatening are curable or controllable.
A new medication must undergo years of research, development, and testing before it can be brought to market. It necessitates both the creation of secure and efficient remedies as well as a thorough comprehension of the biology behind illnesses. We will get a greater understanding of the time and money required to produce drugs that enhance the lives of millions of people as we examine the several phases of pharmaceutical drug manufacture.
The Science of Developing Pharmaceutical Drugs
To appreciate the intricacy of developing new drugs, one must have a solid understanding of the science behind pharmaceutical drug development. From drug development to clinical trials, this procedure entails a number of exacting steps that guarantee the safety and efficacy of new medications.
Drug Discovery and Preliminary Investigations
Researchers find possible pharmacological targets and confirm their function in the illness process during the first phase of pharmaceutical drug development, known as drug discovery. This phase is essential for setting the groundwork for the creation of novel medications.
Identification and Validation of the Target
Finding the biological target—such as a gene or protein—that is connected to a certain illness is known as target identification. Validation verifies that the target is, in fact, relevant to the course of the illness.
Identification of Lead Compounds
Following target validation, scientists look for a lead molecule that may alter the target’s behavior. The foundation for further optimization is this chemical.
Safety Assessment and Preclinical Testing
Preclinical testing evaluates the lead compound’s effectiveness and safety in both lab and animal models. Before the medication is tried on people, this step aids in identifying any problems.
Human Testing and Clinical Trials
In clinical trials, the medicine is tested on people to ascertain its efficacy and safety. These studies are carried out in stages, each of which evaluates a distinct facet of the medication’s functionality.
| Stage | Description | Objective |
|---|---|---|
| Drug Discovery | Identifying potential drug targets and lead compounds | To find a promising compound for further development |
| Preclinical Testing | Assessing safety and efficacy in laboratory and animal models | To evaluate the drug’s potential before human testing |
| Clinical Trials | Testing the drug in humans to determine safety and effectiveness | To confirm the drug’s safety and efficacy in humans |
From drug discovery to clinical trials, there are many steps in the intricate process of developing pharmaceutical drugs. We can grasp the difficulties and complexities involved in developing new drugs if we comprehend the science behind this process.
The Manufacturing Process for Pharmaceutical Drugs
Pharmaceutical medication manufacture is a meticulous procedure that includes many crucial steps, from synthesis to formulation. For the finished product to be safe, efficient, and compliant with regulations, the manufacturing process is essential.

Production of Active Ingredients and Drug Synthesis
medication synthesis is the first stage of pharmaceutical medication manufacture, during which the active pharmaceutical ingredient (API) is created. To produce the required product, a number of chemical reactions and procedures are needed. The quality and purity of the API are guaranteed by the use of cutting-edge technologies and procedures.
Dosage forms and pharmaceutical formulation
Following synthesis, the API is prepared into various dosage forms, including injectables, pills, capsules, and other delivery methods. A number of parameters, including patient compliance, stability, and bioavailability, must be carefully taken into account throughout the formulation process.
Capsules and Tablets
Among the most popular dose forms are tablets and capsules. These are made by combining the API with excipients, then granulating, compressing, and coating the mixture. Every step of the process includes quality control procedures to guarantee that the finished product satisfies the necessary requirements.
Injectables and Additional Methods of Administration
Specialized manufacturing procedures are needed for injectables and other delivery methods, such topical lotions and ointments. These goods need to be sterile and adhere to strict legal specifications. The quality and safety of these items are guaranteed by the use of advanced production technology.
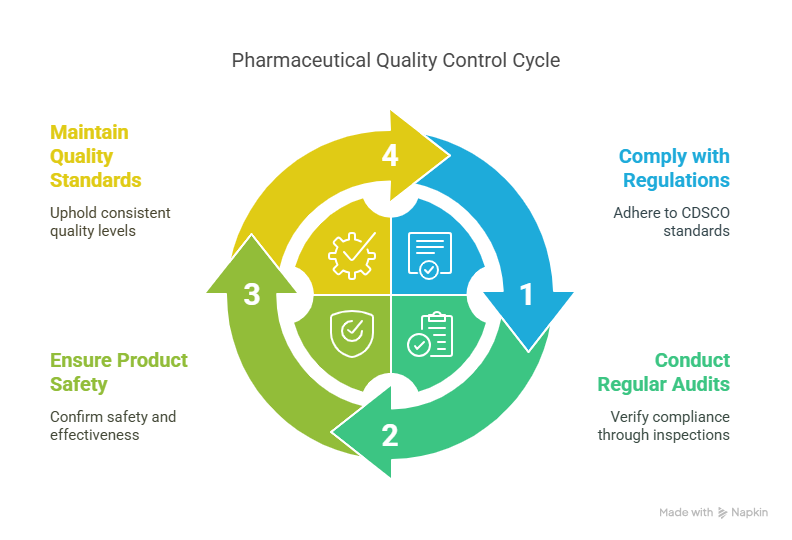
India’s Regulatory Compliance and Quality Control
Pharmaceutical firms operating in India are required to abide by rules established by regulatory bodies like the Central Drugs Standard Control Organization (CDSCO). A crucial component of pharmaceutical production is quality control, which guarantees that the finished product is both safe and effective for ingestion. To make sure that regulatory criteria are being followed, routine audits and inspections are carried out.
Conclusion: From Patient to Production
From research and development to manufacture and distribution, the process of making pharmaceutical drugs is a multi-step, intricate one. Knowing the manufacturing process of pharmaceuticals makes it easier to appreciate the work that goes into making life-saving medicines. Pharmaceutical manufacturing processes are essential to guaranteeing the efficacy and safety of medications.
Keeping abreast of the most recent discoveries and breakthroughs in medication production is crucial as the pharmaceutical business continues to change. This information makes it possible to comprehend the significance of pharmaceutical production and how it affects public health in India.
Using efficient pharmaceutical production processes is essential to providing patients with high-quality drugs. Manufacturers may increase patient outcomes, lower manufacturing costs, and improve medicine effectiveness by using these strategies.
FAQ
What is the first step in the pharmaceutical drug manufacturing process?
The first step is drug discovery and initial research, which involves identifying potential drug targets and validating their role in the disease process through techniques such as target identification and validation, and lead compound identification.
How are active ingredients produced in pharmaceutical manufacturing?
Active ingredients are produced through drug synthesis, a process that involves the use of various chemical reactions and techniques to create the desired compound, which is then used in the production of pharmaceutical drugs.
What is pharmaceutical formulation, and why is it important?
Pharmaceutical formulation is the process of developing the final dosage form of a drug, such as tablets, capsules, or injectables, taking into account factors like bioavailability, stability, and patient compliance, to ensure the safe and effective delivery of the active ingredient.
What are the different dosage forms used in pharmaceutical manufacturing?
The different dosage forms used include tablets, capsules, injectables, and other delivery systems, each with its own advantages and requirements, such as the need for sterile manufacturing processes for injectables.
How do pharmaceutical companies ensure quality control and regulatory compliance?
Pharmaceutical companies ensure quality control and regulatory compliance by adhering to strict guidelines and regulations set by authorities, such as those in India, and implementing quality control measures throughout the manufacturing process, from drug synthesis to final product testing.
What is the role of clinical trials in pharmaceutical drug development?
Clinical trials play a crucial role in testing the safety and efficacy of pharmaceutical drugs in humans, providing valuable data that informs the development process and ensures that only safe and effective drugs reach the market.
How do pharmaceutical manufacturing techniques impact public health?
Pharmaceutical manufacturing techniques have a significant impact on public health by ensuring that drugs are safe, effective, and available to those who need them, ultimately contributing to the improvement of healthcare outcomes and the quality of life.

